New findings about antibiotic resistance of bacteria
October 24, 2017
Why do some antibiotics make no impact on some bacterial diseases – and what could be done against this? Researchers from Newcastle University and Jacobs University shed new light on a machinery which keeps the outer membrane in many bacteria asymmetric. The damage of the sugar-coated layer on the surface of the bacteria would make the bacteria more susceptible to antibiotics. Thus, the findings are a starting point to determine whether the studied system of Gram-negative pathogens could be targeted by drugs to decrease bacterial virulence and to make various antibiotics more effective.
The researchers, led by Professor Bert van den Berg from Newcastle University and including Prof. Ulrich Kleinekathöfer from Jacobs University Bremen, report on their findings in October in Nature Microbiology. The research has received support from the Innovative Medicines Initiatives, a joint undertaking from the European Union and the European Federation of Pharmaceutical Industries and Associations, through the project "Translocation" of the “New Drugs for Bad Bugs” platform.
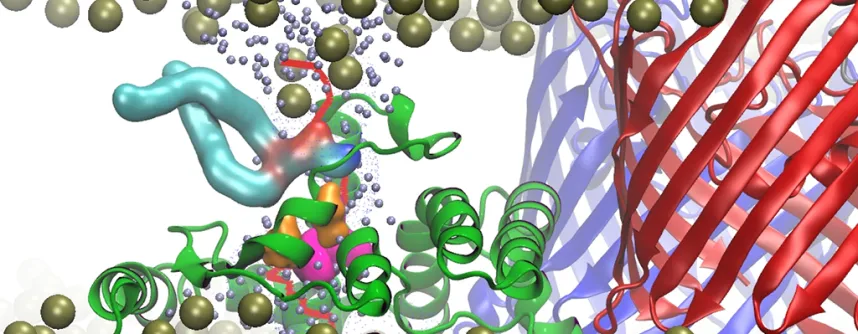
Their findings refer to so-called Gram-negative bacteria, which differ from Gram-positive bacteria by the structure of their cell wall. Gram-negative bacteria such as E. coli have two membranes, an inner and an outer membrane. The outer one is an asymmetric bilayer with an inner leaflet of phospholipids and an outer leaflet composed almost exclusively of lipopolysaccharide (LPS). The LPS forms a sugar-coated layer on the surface of Gram-negative bacteria that is a very effective barrier for greasy, hydrophobic molecules and causes resistance towards antibiotics and other harmful compounds. Thus, from the bacterium's point of view, the asymmetry of the outer membrane is very important. However, phospholipids spontaneously accumulate in the outer leaflet of the outer membrane, forming "islands" amid the LPS that increase the outer membrane permeability of toxic compounds. Those phospholipid molecules need to be removed from the outer leaflet and asymmetry restored. This process is carried out by the Mla (maintenance of lipid asymmetry) system, which is present in most Gram-negative bacteria. The MlaA protein, the focus of the study, is the outer membrane component of the Mla system.
The scientists at Newcastle University today report that they have determined the first three-dimensional atomic structures of the MlaA protein by X-ray crystallography. Based on this structural data, the Kleinekathöfer group from Jacobs University performed molecular simulations. The research of the scientists from Bremen shows: the donut-shaped MlaA binds phospholipids from the outer leaflet and removes these via a central channel, somewhat analogous to a vacuum cleaner. “We are very happy that our knowledge as computational biophysicists might help to find new drug targets and to make present antibiotic more potent again”, Kleinekathöfer states.
Additional information at:
Reference: Structural basis for maintenance of bacterial outer membrane lipid asymmetry: http://dx.doi.org/10.1038/s41564-017-0046-x
http://ukleinekat.user.jacobs-university.de/
Questions will be answered by:
Prof. Dr. Ulrich Kleinekathöfer | Professor of Theoretical Physics
u.kleinekathöfer [at] jacobs-university.de | Tel.: +49 421 200- 3523