

Prof. Dr. Nikolai Kuhnert
Analytical Chemistry, Mass Spectrometry, Natural Product Chemistry, Food Chemistry, Food Processing, Cocoa, Coffee, Black Tea
Research in the Kuhnert group investigates the food we eat every day. Following Hippocrates stating that “Food be thy medicine and medicine be thy food” we try identify the chemical compounds in our daily diet and link them to beneficial and detrimental health effects. We have laid a particular focus on dietary plants rich in polyphenols (coffee, black tea, cocoa, red wine) over the last two decades.
In particular we focus on processed food. Food processing is a uniquely human activity that distinguishes us from all other species on our planet. Food processing includes mainly thermal treatment (cooking, baking, frying, steaming etc) or microbial treatment or fermentation. During food processing the chemical composition of the food raw material changes dramatically. Most of the primary and secondary metabolites present in the raw material are converted to novel chemical compounds, a true terra incognita. Following food processing the chemical compounds in our food encounter the gut microbiota, around 1000 different species of microorganisms that again change the chemical composition of our diet. Finally compounds reach following absorption the liver where liver enzymes again catalyse chemical changes. Hence to understand any health effect of a given food all these three steps must be investigated in detail to enhance our understanding of the role of our diet.
To carry out this enormously complex task we mainly rely on modern mass spectrometrical techniques combined with the powerful potential of big data chemometrics and bioinformatics. Ur research is driven by academic curiosity in combination with applied research in cooperation with partners from the food industry.
For more detailed information please visit the following subsections
Polyphenols are ubiquitous plant secondary metabolites. Whether you drink coffee, red wine or tea or enjoy fruits, berries, vegetables or chocolate you take in gram quantities every day of this class of compounds. For the general public these compounds are better known as antioxidants.
From decades of research it is evident beyond all doubt that polyphenols are beneficial for human health. Epidemiological studies show that population groups consuming high levels of polyphenols are healthier, suffer less from cancer, cardiovascular disease, diabetis etc and age slower. Clinical intervention studies have added to this knowledge, when volunteers consumed a diet rich in polyphenols and their health parameters were monitored. Sometimes this effect is rather small and debatable (coffee and cancer, red wine and long life, cocoa and cardiovascular health) sometimes it is dramatic (coffee and diabetis). In our research group we investigate the chemistry of polyphenols with a particular emphasis on their health effects and the underlying mechanisms. At the current state we can summarise that we do not understand the mechanisms behind the relationship between polyphenols and health, however we can conclude that it is not the compounds present in our diet but rather their gut microbial metabolites and human hepatic metabolites that must cause a multitude of beneficial health effects.
Scientific progress is achieved by new technologies, discoveries and new ideas, probably in that order. (Sydney Brenner)
Scientific progress is, like it or not, achieved by new technologies. New technologies provide unprecedented capabilities that change our view of nature and lead to new ideas and concepts.
Mass spectrometry, which dramatically evolved and changed over the last two decades, constitutes in our view a technique that has and will fundamentally change the chemical and life sciences offering powerful capabilities driving future scientific progress. These capabilities in order of importance are:
1. Unsurpassed Resolution
A modern high-resolution mass spectrometer can resolve several thousand analytes concomitantly and at the current state of art ultra high-resolution instruments can resolve up to 100 000 analytes in a single spectrum. This resolution is several orders of magnitude higher than any other spectroscopic or separation technique, providing unprecedented insight into the nature and composition of matter. If we assume that a human organism is made up of around 100 000 different chemical entities, this means that at least in theory all human molecules can be analyzed at the same time in a single measurement.
2. Structural Information
MS provides two levels of structural information. Firstly resolution MS data provide information on molecular formulas (elemental composition) and secondly tandem MS provides via fragmentation further structural information on bond connectivities.
3. Coupling to separation techniques
MS instruments can be routinely interfaced with separation devices including HPLC or GC instrument. Additionally, further spectrometers can be interfaced providing for each analyte, multi-dimensional specificity, and a multitude of structural information via measurement of retention time UV-VIS absorption and MS data.
4. Sensitivity
MS is one of the most sensitive techniques available to chemists with routine sensitivities in the fmol region. Theoretical considerations predict a maximum sensitivity of around 10 ions, which we might see realized within the next decades.
5. Versatility
MS can be used for any type of analyte independent of its size, physical state (gas, solid, or liquid) or sensitivity. Modern ionization techniques allow the generation of ions from any analyte imaginable.
6. Speed and Ease of Analysis
MS techniques are relatively easy to use (a few months of training is sufficient) are rapid and amenable to high-throughput applications.
In our research, we use many aspects of modern mass spectrometry to study food, biological systems or natural or synthetic compounds. We try to make the best use of its unique capabilities and try to identify challenges and find solutions to the challenges to improve the capabilities of this technique to advance scientific progress.
The main challenges in modern mass spectrometry are the following, which we try to address in our research:
1. Interpretation of complex data
As mentioned before, modern high-resolution MS can routinely provide spectra with tens of thousands of different ions present in a single spectrum. How do we interpret such data? Finding solutions to obtain chemical or biological meaningful information is one of MS most pressing and urgent challenges. We have adopted methods devised in petroleomics to address such complex samples and developed a series of novel data interpretation strategies to extract chemical and biological relevant information from such enormously complex data.
2. Obtaining structural information
We believe that MS is not living up to its full capabilities in chemical structure elucidation. Despite many advances, deriving a full chemical structure from MS data is currently only possible in exceptional circumstances. We believe this can be changed by achieving a better understanding of fragmentation mechanisms and using more sophisticated tandem MS techniques such as energy resolved mass spectrometry. Work in this field will be published soon.
3. Distinction of and unambiguous characterization of isomers
Generally MS is considered to be isomerically blind. Isomers, compounds of identical molecular formula, are generally believed to show if any, only subtle differences in their MS spectra. This is not true. We have shown for a series of regioisomeric compounds (chlorogenic acids, shikimic acid derivatives, carbohydrates) that the use of tandem MS techniques provides a powerful method to distinguish isomeric compounds and even allows a reliable prediction of chemical structure (in most cases even superior to NMR). Current work focuses on the extension of these methods and the distinction of stereoisomers by tandem MS.
4. Ion Suppression and Ion Enhancement
If more than one analyte is present in an MS analysis, these analytes compete for ionization, leading to a reduction or enhancement of any number of signals. This effect has been termed ion suppression or enhancement and next to spoiling any quantitation attempts is to the current day poorly understood. Understanding ion enhancement is of utmost importance in complex mixture analysis. If thousands of analytes are present we must know, which ones we can observe and which ones not and what level of quantitative information we can derive from such experiments. We are addressing the problem of ion suppression and have recently shown that such effects can be rationalized based on the chemical structure itself.
5. Quantification
For quantification in MS authentic reference materials are required, ideally a set of isotopically labeled and unlabeled reference material (because of ion suppression) to allow thorough quantification of analytes. Would it not be great if quantification could be carried out without such a requirement? Current work is addressing this problem trying to find methods that allow reference free MS quantification.
6. Non-covalent interactions
In MS both non-covalently bound and covalently bound species can be analyzed. In several ongoing projects, we try to understand the nature of non-covalent interactions in MS to be able to study both interactions in biological systems.

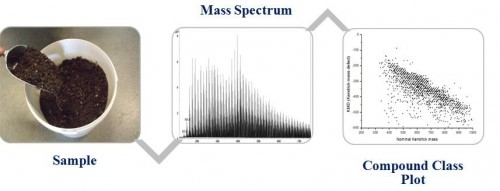
Every human being is a complex mixture of around 100,000 different chemicals and as humans, we eat and are surrounded by vast complex mixtures of additional millions of chemical compounds affecting our daily lives. We define a complex mixture as a mixture that contains too many individual compounds to allow separation by chromatographic methods (more than 1000 in GC and more than 300 in LC).
Analysing such complex mixtures forms the ultimate challenge of analytical chemistry and life sciences but the rewards will be tremendous. Chemists have by tradition detested such mixture analysis and reduced the world to the analysis of single purified and well-defined compounds. Modern mass spectrometry, with its unsurpassed resolution, allows, however, the simultaneous analysis of tens of thousands of compounds in a single experiment, which in chromatography are manifested as an unresolved complex hump. In our research, we apply novel measurement strategies and data interpretation strategies to study such complex mixtures in food, biological systems, and environmental samples trying to develop tools allowing an understanding and a chemically meaningful interpretation of data generated from complex mixtures. The steps of our analysis strategy comprise the following steps:
- High resolution MS studies (provide a list of thousands of molecular formulas)
- Conventional spectroscopic characterization (NMR, IR, UV-VIS, Raman, and CD-spectroscopy provide a global picture of the compounds present in the absence of resolution)
- New data interpretation tools such as van Krevelen and Kendrick analysis (borrowed from petroleomics), unsaturation analysis, or homologous series analysis (the idea is that in nature only a set of a few reaction mechanisms and building blocks are used, which can be extracted from such datasets)
- Formulation of a structural and compositional hypothesis
- Testing this hypothesis by any spectroscopic method suitable e.g. LC-tandem MS in our black tea work.
- As an alternative Data reduction (Principal component analysis). Here we probe which parameters are important in the data set and ignore everything else.
Complex Mixtures in Food
Food contains many different compounds and is a highly complex material. Its complexity increases dramatically if man subjects food to harsh unfriendly conditions such as roasting, cooking, baking, frying, steaming, or fermentation.
Examples of our work in the area include the analysis of black tea thearubigins, coffee polyphenols, and recent work on cocoa polyphenols.
Following consumption of processed food, compounds of medium polarity are absorbed in the small intestine. All polar compounds reach the colon, where they are metabolized by the gut microbiota to produce a myriad of microbial metabolites. Some of them are absorbed and enter human circulation, where they are transported to the liver and subjected to Phase I and Phase II metabolism.
Over the last five years, we have carried out three human volunteer studies looking at the fate of cocoa, coffee and black tea constituents in human body fluids and feces using high-resolution LC-tandem MS methods. Some of the work is published and further studies are planned. As a preliminary conclusion we observe that the majority of compounds observed in human urine are metabolites of dietary compounds, most of them with an unknown structure. Identification of metabolites linked to the consumption of a certain food requires sophisticated data interpretation approaches since unknown compounds need to be identified, in a complex mixture of thousands of other unrelated compounds. To solve this issue, we introduced two novel approaches, tandem MS molecular networking to link unknown metabolites to known structures based on similarities of fragment spectra and filtering of multivariate statistical data based on absorption times and dosages.
1987-1993: Undergraduate Studies in Chemistry at the University of Würzburg, Germany
1993-1995: Ph.D. with W. A. Schenk, Universität Würzburg Thesis: “Synthesis, Reactivity and Dynamic Behaviour of Rutehnium Sulfine Complexes”
1996/1997: Postdoc with S. Warren at the University of Cambridge, UK
1997/1998: Postdoc with Jeremy Robertson at the University of Oxford, UK
1997/1998: Tutor in organic chemistry at Hertford College and Stanford Centre (Keble College), Oxford, UK
1998-2005: Lecturer in organic chemistry at the University of Surrey, Guildford, UK
2005/2006: Senior Lecturer in organic chemistry at the University of Surrey, Guildford, UK
since 2006: Full Professor of Chemistry at the Jacobs University Bremen
Member of the Society of German Chemists (GDCh)
Fellow of the Royal Society of Chemistry (FRSC)
Member of the Deutsche Hochschulverein (DHV)
Member of the DGMS (Deutsche Gesellschaft für Massenspektroskopie)
President of the Surrey Chemical Society (2001/2006)
Groupe Polyphenole
Visiting Professor at the Universität des Saarlandes (2000)
Visiting professor (Innovartec Chair for combinatorial chemistry), Universität Regensburg (2001)
Visiting Professor of Chemistry at the Jacobs University Bremen (2006)
Sabbatical at CSIC Rocasolano in Madrid (Spain) with Prof. Juan Davalos (2012)
- Profiling of Regioisomeric Triacylglycerols in Pistachio Nuts by High-Performance Liquid Chromatography-Electrospray Ionization Mass Spectrometry“, Sabzi, F.; Sirbu, D.; Kuhnert, N. J. Food Compos. Anal. 2023, 122. https://doi.org/10.1016/j.jfca.2023.105395.
- “Lignin and Its Pathway-Associated Phytoalexins Modulate Plant Defense against Fungi”, Ninkuu, V.; Yan, J.; Fu, Z.; Yang, T.; Ziemah, J.; Ullrich, M. S.; Kuhnert, N.; Zeng, H.. J. Fungi 2023, 9 (1). https://doi.org/10.3390/jof9010052.
- „PtIV-Containing Hexaplatinate(II) [PtIVPtII 6O6(AsO2(CH3)2)6]2- and Hexapalladate(II) [PtIVPdII6O6AsO.”, Zhang, J.; Bhattacharya, S.; Khsara, B. E.; Nisar, T.; Müller, A. B.; Besora, M.; Poblet, J. M.; Wagner, V.; Kuhnert, N.; Kortz, U. Inorg. Chem. 2023. https://doi.org/10.1021/acs.inorgchem.3c00832.
- “Rationalising the Retro-Diels-Alder Fragmentation Pattern of Viscutins Using Electrospray Interface-Tandem Mass Spectrometry Coupled to Theoretical Modelling.”, Moyo, B.; Novokoza, Y.; Tavengwa, N. T.; Kuhnert, N.; Lobb, K.; Madala, N. E. Rapid Commun. Mass Spectrom. 2023, 37 (15). https://doi.org/10.1002/rcm.9592.
- “ Mixed-Valent Palladium(Iv/Ii)-Oxoanion, [PdIVO6PdII6((CH3)2AsO2)6]2”, Ma, X.; Bhattacharya, S.; Nisar, T.; Müller, A. B.; Wagner, V.; Kuhnert, N.; Kortz, U., Chem. Commun. 2023, 59 (7), 904–907. https://doi.org/10.1039/d2cc05699b.
- “Understanding the fragmentation of glucose” M. Patras, J. Z. Davalos and N. Kuhnert, J. Mass Spectrom. 2023, manuscript accepted, in press.
- “Unbiased and biased chemometric analysis of LC-MS data from human urine following coffee intake”, I. Said, S. Haka, J. Truex, C. heidorn, D. Petrov and N. Kuhnert, , J. Mass Spectrom. 2023, manuscript accepted, in press.
- “Identification of oxygenated triacylglycerols in pistachio nuts” F. Sabzi and N. Kuhnert, Food Res. Int. 2023, manuscript accepted.
- Clifford, M. N.; Kuhnert, N. LC–MS Characterization and Quantification of Known and Unknown (Poly)Phenol Metabolites—Possible Pitfalls and Their Avoidance. Mol. Nutr. Food Res. 2022. https://doi.org/10.1002/mnfr.202101013.
- “A practitioner’s dilemma – Mass Spectrometry-Based Annotation and Identification of Human Plasma and Urinary Polyphenol Metabolites” N. Kuhnert and M. Clifford, Mol. Nutr. Food Res. 2022. https://doi.org/10.1002/mnfr.202100985.
- “Investigating the interaction between dietary polyphenols, the human ACE-2 receptor and the SARS CoV-2 Spike Protein” D. Schmidt, N. Ohl, P. Cotrell and N. Kuhnert, Food Func. 2022, https://doi.org/10.1039/d2fo00394e.
- "Discrete, Cationic Palladium(II)-Oxo Clusters via f-Metal Ion Incorporation and Their Macrocyclic Host-Guest Interactions with Sulfonatocalixarenes", S. Bhattacharya, A. Barba-Bon, T.A. Zewdie, A. Müller, I. A. Rutkowska, V. Wagner, N. Kuhnert, W. Nau and U. Kortz, Angew. Chemie - Int. Ed. 2022. https://doi.org/10.1002/anie.202203114.
- " LC–MS Characterization and Quantification of Known and Unknown (Poly)Phenol Metabolites—Possible Pitfalls and Their Avoidance" N. Kuhnert and M. Clifford, Mol. Nutr. Food Res. 2022. https://doi.org/10.1002/mnfr.202101013.
- "Cocoa Bean Fingerprinting via Correlation Networks", S. Kumar, R. N. d'Souza, M. Corno, M. Ullrich, N. Kuhnert, M.-T. Hütt, npj Sci. Food 2022, 6 (1). https://doi.org/ 10.1038/s41538-021-00120-4.
- “Cocoa origin classifiability through LC-MS data: A statistical approach for large and long-term datasets” S. Kumar, R. N. d’Souza, B. Behrends, N. Kuhnert, M. Ullrich, M.T. Hütt, Food Res. Int. 2021, 140, 109983.
- “Editorial special edition Cocotea 2019: Fifth international conference on coffee, cocoa and tea” N. Kuhnert and M. Ullrich, Food Res. Int. 2021, 143, 10243.
- “Thermal peroxidation of dietary pentapeptides yields N-terminal 1,2 dicarbonyls” M. Bikaki and N. Kuhnert, Frontiers Nutrition, 2021, 8, 663233.
- “Heat induced cleavage of the peptide bond in dietary peptides and proteins in food processing” M. Bikaki, R. Shah, A. Müller and N. Kuhnert, Food Chem. 2021, 357, 129621.
- “HPLC-MS based design of experiments approach on cocoa roasting” P. Andruszkiewicz, R. d’Souza, M. Corno and N. Kuhnert, Food Chem. 2021, 360, 129694.
- “LC-MS based metabolomic approach for the efficient identification and relative quantification of bioavailable polyphenolic metabolites in human urine” I. Said, C. Heidorn, D. Petrov, J. Truex, M. Retta, S.Haka, M. Ullrich and N. Kuhnert, Food Chem. 2021, 364, 130198.
- “Small peptides in cocoa” N. Kuhnert, Sweet Visions, 2, 2021, 2-4.
- “Changes of low molecular weight carbohydrates in kale (Brassica oleraceae) during development and acclimation to cold temperatures determined by chromatographic techniques coupled to mass spectrometry” R. Megias-Perez, C. Hahn, B. Behrends, D. Albach and N. Kuhnert, Food Res. Int. 2020, 127, 108865-108875.
- “Naturstoffe aus Rhododendren als Quelle neuer Antibiotika” Rhodoendron und Immergrün, N. Kuhnert, M. Ullrich, D. Albach, H. Schepker, I. Said, A. Shrestha, A. Rezk und J. Noelzen, 2020, Band 29, 3-14.
- “Monitoring the changes of low molecular weight carbohydrates in cocoa beans during spontaneous fermentation: A chemometric and kinetic approach” R. Megias-Perez, M. Zambrano-Moreno, M. Behrends, M. Corno and N. Kuhnert, Food Res. Int. 2020, 127, 108865-108875.
- “LC-MS/MS based molecular networking approach for the identification of cocoa phenolic metabolites in human urine” I.H. Said, J. Truex, S. Haka, D. Petrov, M. Retta, C. Heidorn and N. uhnert, Food Res. Int. 2020, 128, 109119-109129.
- “Investigating time dependent cocoa bean fermentation by ESI-FT-ICR mass spectrometry” N. Kuhnert. R. N. d’Souza, M. Witt and M. Ullrich, Food Res. Int. 2020, 128, 109209-109219.
- “Classification of Brazilian roasted coffee beans from different geographical origins and farming practices based on chlorogenic acid profiles”, S. Badmos, M. Fu, D. Granato and N. Kuhnert, Food Res. Int. 2020, 134, 109218-109228.
- “Evaluation of carbohydrates and quality parameters in six types of commercial teas by targeted statistical analysis” A. Shevchuk, R. Megias-Perez, Y. Zemedie and N. Kuhnert, Food Res. Int. 2020, 133, 109122.
- “Novel Amadori and Heyns compounds derived from short peptides found in dried cocoa beans” P. Andruszkiewicz, R. N. d’Souza, M. Corno and N. Kuhnert, Food Res. Int. 2020, 133, 109164.
- “Functional changes induced by extrusion during cocoa alkalization” D. Valverde, B. Behrends, É. Pérez-Esteve, N. Kuhnert and J. M. Barat, Food Res. Int. 2020, 136, 109469.
- “Recommendation for standardizing nomenclature for dietary (poly)phenol catabolites” C. D. Kay, M. Clifford, P. Mena, D. del Rio, C. Andres-Lacueva, C. Manach, D. S. Wishart, G. Pereira-Caro, F. Tomas-Barberan, N. Kuhnert, G. Williamson and A. Crozier, Amer. J. Clin. Nutrition, 2020, 00, 1-18.
-
“Inonenmobilitätsmassenspektrometrie in der Analyse isomerer Lebensmittelinhaltsstoffe” G. H. Yassin and N. Kuhnert, Deutsche Lebensmittelrundschau, 2015, 12, 687-682.
-
“Differentiation of prototropic ions in regioisomeric caffeoyl quinic acids by electrospray ion mobility mass spectrometry” N. Kuhnert, G. H. Yassin, R. Jaiswal, M. F. Matei and C. Grün, Rapid Commun. Mass Spectrom. 2015, 29, 675-680.
-
“Photochemical isomerisations of chlorogenic acids in model systems and in agricultural practice” H. Karaköse, R. Jaiswal, S. Deshpande and N. Kuhnert, Food Chem. 2015, 63, 3338-3347.
-
“Characterization of Microalgal Biocrude Obtained by Hydrothermal Liquefaction of Nannochloropsis salina using Ultra High Resolution APCI Fourier Transform Ion Cyclotron Resonance Mass Spectrometry”, M. M. Sanguinetti, N. Hourani, M. Mathery, M. Witt, L. Thomsen, N. Kuhnert, Rapid Commun. Mass Spectrom., 2015, 1255-1264.
-
“A model system for the mechanism of black tea thearubigin formation “ G. H. Yassin, J. Koek and N. Kuhnert, Food Chemistry, 2015, 180, 272-279.
-
“The fascination of black tea chemistry” Deutscher Teeinformationsservice, deutscher Teetrinkerverband, April 2015.
-
“Profiling and quantitation of phenolics in stevia rebaudiana leaves” H. Karaköse, A. Müller and N. Kuhnert, J. Food Agr. Chem. 2015, 63,9188-9198 .
-
“Synthesis of tetramethoxy-(tetra-hydrazinecarboxamide) cyclophanes with unexpected conformation, and investigation of their solution-phase recognition of chiral carboxylic guests using time-of-flight and tandem mass spectrometry” H. F. Nour, A. Golon, T. El Malah and N. Kuhnert, , ARCIVOC, 2015, 5, 1-19.
-
“UHPLC-Q-TOF-MS characterization of leaf extracts of hawthorn (Crataegus)”M. E. Karar and N. Kuhnert, J. Biological Chem. Ther. 2015, 3, 3411-3425.
-
“A review on Sudanese traditional medicinal plants”, M. E. Karar and N. Kuhnert, Rev. Pharmacognosy 2015, manuscript in press.
-
“Quantification of microbial uptake of quercetin and its derivatives using an UHPLC-ESI-QTOF mass spectrometry assay”. I.H. Said, R.L. Shah, M.S. Ullrich and N. Kuhnert Food Funct., 2016, 7, 4082-4091.
-
“Aseptic artificial fermentation of cocoa beans can be fashioned to replicate the peptide profile of commercial cocoa bean fermentations”. W. A. John, N. Kumari, N. L. Böttcher, K. J. Kofi, S. Grimbs, G. Vrancken, R. N. D’Souza, N. Kuhnert, and M. S. Ullrich, Food Research International 2016, 89, 764–772.
-
“Biochemical fate of vicilin storage protein during fermentation and drying of cocoa beans”. N. Kumari, K. J. Kofi, S. Grimbs, R. N. D’Souza, N. Kuhnert, G. Vrancken, and Ullrich, M. S., Food Research International 2016, 90, 53–65.
-
“Diversity of kale (brassica oleraceae var. sabellica) Glucosinolate content and phylogenetic relationships”. C. Hahn, A. Müller, D. Albach and N. Kuhnert, Agr. Food Chem., 2016, 64, 3215-3225.
-
“Polyphenol profile and antibacterial activity of Ziziphus Spinae Christi extracts”, M. E. Karar, M. Ullrich, K. Brix, R. Jaiswal and N. Kuhnert, Omics J. Med. Chem., 2016, 1, 345-356.
-
“Neuroaminidase inhibition of dietary chlorogenic acids and derivatives – Potential antivirals from dietary sources”, Food Function, 2016, 7, 2052-2059.
-
“Bioactivity in Rhododendron: A systemic analysis of antimicrobial and cytotoxic activities and their phylogenetic and phytochemical origins”. A. Grimbs, A. Shrestha, A.S. Rezk, S. Grimbs, I. Hakeem Said, H. Schepker, M.T. Hütt, D.C. Albach, K. Brix, N. Kuhnert and M.S. Ullrich. Frontiers in plant science, 2017, 8.
-
“Origin-based polyphenolic fingerprinting of Theobroma cacao in unfermented and fermented beans”. R. N. D’Souza, S. Grimbs, B. Behrends, H. Bernaert, M. S. Ullrich, and N. Kuhnert, Food Research International 2017, http://dx.doi.org/10.1016/j.foodres.2017.06.007
-
“Metabolome Comparison of Bioactive and Inactive Rhododendron Extracts and Identification of an Antibacterial Cannabinoid(s) from Rhododendron collettianum”, I. Hakeem Said, A. Grimbs, A. Shrestha, K. Brix, M. Ullrich, N. Kuhnert, Analysis, 2017, http://dx.doi.org/10.1002/pca.2694
-
“Profiling and quantification of regioisomeric caffeoyl glucoses in Solanaceae vegetables”, M. A. Patras, R. jaiswal, N. Kuhnert, Food Chem. 2017, 237, 659-666.
-
“Comparison of the polyphenolic profile and antibacterial activity of the leaves, fruits and flowers of Rhododendron ambiguum and Rhododendron cinnabarinum”, A. Shrestha, A.S. Rezk, I. Hakeem Said, V. Glasenapp, R Smith, M.S. Ullrich, H. Schepker, N. Kuhnert, BMC Research Notes, 2017, 10 (1), 297.
-
“Determination of hydroxycinnamic acids present in Rhododendron species”, A. Shrestha, I. Hakeem Said, N. Thielen, L. Lansing, H. Schepker, N. Kuhnert, Phytochemistry , 2017, 144, 216-225.
-
“Herbal drugs from Sudan: Traditional uses and phytoconstituents – A review”, M. E. Karar and N. Kuhnert, Pharmacognosy Rev. 2017, 11, 83-103.
-
“Leaves metabolomic profiling of Musa acuminata accessions using UPLC–QTOF–MS/MS and their antioxidant activity” M. A. Sonibare, I. O. Ayoola, B. Gueye, M. T. Abberton, R. D’Souza, N. Kuhnert J. Food Meas. Char. 2018 12:1093–1106.
-
“Antibiotika aus Rhododendren” H. Schepker, K. Brix, M. Ullrich and Nikolai Kuhnert, Grüne Forschung, 2018, 42.
-
“Pilot scale production of antibacterial substances by the marine diatom Phaeodactylum tricornutum Bohlin”, S. Wang, I. Hakeem Said, C. Thorstenson, C. Thomsen, M. S. Ullrich, N. Kuhnert and L. Thomsen, Algae Res. 2018, 32, 112-120.
-
“Profiling, quantification and classification of cocoa beans based on chemometric analysis of carbohydrates using hydrophilic liquid interaction chromatography coupled to mass spectrometry”, R. Megias-Perez, R. D’Souza, S. Grimbs, H. Bernaert and N. Kuhnert, Food Chem. 2018, 258, 284-294.
-
“Profiling and quantification of caffeoyl glucoses in berry fruits”, M. A. Patras, R. Jaiswal, G. MacDougal and N. Kuhnert, J. Agr. Food Chem., 2018, 66, 1096-1104.
-
“Tea and coffee with bacteria –Investigation of uptake of key coffee and tea phenolics by wild type E. coli”, I. Hakeem Said, M. S. Ullrich and N. Kuhnert, Food Res. Int. 2018, 108, 584-594.
-
“Energy resolved mass spectrometry of chlorogenic acids and direct infusion regioisomer quantification” J. Hernandez, A. Müller, R. Jaiswal, J. Z. Davalos, N. Kuhnert, Phytochem. Anal. 2018, https://doi.org/10.1002/pca.2770.
-
“Degradation of cocoa proteins into oligopeptides during spontaneous fermentation of cocoa beans” R. N. D’Souza, S. Grimbs, B. Behrends, M. Corno, M. S. Ullrich and N. Kuhnert, Food Res. Int. 2018, 109, 506-516.
-
“Differentiation of black tea infusions according to origin, processing and botanical varieties using multivariate statistical analysis of LC-MS data”, A. Shevchuk, L. Jayasinghe and N. Kuhnert, Food Res. Int. 2018, 109, 387-402.
-
“Origin and varietal based proteomic and peptidomic fingerprinting of Theobroma cacao in non-fermented and fermented cocoa beans” N. Kumari, A. Grimbs, R. N. D’Souza, S. K. Verma, M. Corno, N. Kuhnert and M. S. Ullrich, Food Res. Int. 2018, 111, 137-147.
-
“Characterization of triacylglycerols in unfermented cocoa beans by HPLC-ESI mass spectrometry” D. Sirbu M. Corno M S.Ullrich N. Kuhnert, Food Chem. 2018, 254, 232-240.
-
“Chemistry, antibacterial effects and bioinformatics of Rhododendron secondary metabolites, I. Hakeem Said, M. S. Ullrich and N. Kuhnert, Rhododendron Int. 2018, article in press.
-
“Changes in the fucoxanthin production and protein profiles in Cylindrotheca closterium in response to blue light-emitting diode light” S. Wang, S. K. Verma, I. Hakeem Said, L. Thomsen, M. S. Ullrich and N. Kuhnert Microbial Cell Factories 2018 17:110.
-
“Combined use of gas chromatography and HILIC chromatography coupled to mass spectrometry for the characterization and quantification of unknown carbohydrates in cocoa beans” R. Megias-Perez, A. I. Ruiz-Matute, M. Corno, A. Grimbs and N. Kuhnert, J. Chromat. A. 2019, 135-143.
-
“Biological activities of Ficus carica latex for potential therapeutics in human Papillomavirus (HPV) related cervical cancers” A. Ghanbari, A. LeGresley, D. Naughton, N. Kuhnert, D. Sirbu and G. H. Ashrafi, Nature Sci. Rep. 2019, 9, 1013-1017.
-
“Profiling and quantification of carbohydrates in green tea infusions” R. Megias-Perez, Y. Zemedie, A. Shevchuk and N. Kuhnert, Food Chem., 2019, 290, 159-167.
-
“Thermally-induced formation of taste-active 2,5-diketopiperazines from short-chain peptide precursors in cocoa” P. J. Andruszkiewicz, R. d’Souza, I. Altun, M. Corno and N. Kuhnert, Food Res. Int. 2019, 121, 218-228.
-
“Thermal degradation of Peptides by decarboxylation and tryptohane oxidation” M. Bikaki and N. Kuhnert, J. Agr. Food Chem. 2019, 67, 7448-7454.
-
“Comparison and quantification of chlorogenic acids for differentiation of green Robusta and Arabica coffee beans” S. Badmos, S. H. Lee and N. Kuhnert, Food Res. Int. 2019, 126, 108544.
-
“Experimentally modelling cocoa bean fermentation reveals key factors and their influences” W. A. John, N. L. Böttcher, B. Behrends, M. Corno, R. d’Souza, N. Kuhnert and M. S. Ullrich, Food Chem, 2019, 302, 12535.
-
“Comparative lipidomic studies of Scenedesmus sp. (Chlorophyceae) and Cylindrotheca closterium (Bacillariophyceae) reveal their differences in lipid production under nitrogen starvation” W. Song, D. Sirbu, L. Thomsen and N. Kuhnert, J. Phycol. 2019, manuscript in press.
-
On the chemistry of small molecular weight polyphenols in black tea, J. W. Drynan, M. N. Clifford, J. Obuchowicz and N. Kuhnert, Prod. Rep.2010, 27, 417-462. read online
-
The design and synthesis of deep cavity tetra-acrylato-imine calix[4]arene for the development of static and dynamic combinatorial libraries, N. Kuhnert and A. Le-Gresley, Chem. Res., 2010, 2,61-67.
-
Repeated oral administration modulates the pharmacokinetic behaviour of the chemopreventive agent phenylethyl isothiocyanate in rats, N. Konsuye, N. Kuhnert, J. Kirkpatrick, L. J. King and C. Ioannides, Nutr. Food Res. 2010, 64, 426-432. read online
-
How to distinguish feruloyl from isoferuloyl quinic acids by tandem mass spectrometry, R. Jaiswal, M. Matei, S. Deshpande, T. Sovdat, N. Kuhnert, Rapid Commun. Mass Spectrom. 2010, 24, 1575-1582. read online
-
Profiling the chlorogenic acids and hydroxyl cinnamoylshikimates in mate (ilex paraguayensis), R. Jaiswal, T. Sovdat, N. Kuhnert, Agr. Food Chem. 2010, 58, 5471-5484. read online
-
Analysis, characterization and pharmacokinetics of dietary hydroxycinnamates, N. Kuhnert, H. Karaköse, R. Jaiswal, Invited review chapter in CRC Handbook of Food Analysis, manuscript in press.
-
Hierarchical scheme for the identification of 3,4,5 triacyl chlorogenic acids in Robusta green coffee beans,Rapid Commun. Mass Spectrom. 2010, 24, 2283-2294. read online
-
Profiling the chlorogenic acids of Stevia Rebaudiana by tandem LC-MS, H. Karaköse and N. Kuhnert,Polyphenol Commun. 2010, Vol 1, 544-546. 71.
-
Analysis of the chlorogenic acids and hydroxycinnamoyl shikimate esters in green tea and mate tea by LC-MSn, R. Jaiswal, T. Sovdat, M. Patras and N. Kuhnert, Polyphenol Commun. 2010, Vol 1, 536-538.
-
Unravelling the structures of black tea thearubigins, N. Kuhnert, Polyphenol Commun. 2010, Vol 1, 56-58.read online
-
Profile and Characterization of the Chlorogenic Acids in Green Robusta Coffee Beans by LC-MSn –Identification of Seven New Classes of Compounds, R. Jaiswal, P. Eruchivera, M. A. Patras and N. Kuhnert, Agr. Food Chem.2010, 58, 8722-8737. read online
-
Unravelling the structure of black tea thearubigins, N. Kuhnert, Biochem. Biophys. 2010, 501, 37-51.
-
Profiling the Chlorogenic Acids of Rudbeckia hirta, Helianthus tuberosus, Carlina acaulis, and Novae angliae Leaves by LC-MS, R. Jaiswal, S. Deshpande and N. Kuhnert, Phytochem. Anal. Manuscript accepted.read online
-
On the chemical characterization of black tea thearubigins using mass spectrometry, N. Kuhnert, J. W. Drynan, J. Obuchowicz and M. Clifford, Rapid Commun. Mass Spetrom. Manuscript accepted.
-
Identification and characterization of five new classes of chlorogenic acids containing aliphatic side chains in Gardenia fructis (Gardenia sativa) by liquid chromatography tandem mass spectrometry, R. Jaiswal, M. N. Clifford and N. Kuhnert, Rapid Commun. Mass Spectrom. manuscript accepted.
-
Analysis of black tea thearubigins: Evidence for oxidative cascade reactions forming the thearubigins, N. Kuhnert, M. N. Clifford and A. Müller, Food and Function, manuscript accepted 2010.
-
Identification and characterization of five new classes of chlorogenic acids containing aliphatic side chains in Burdock (Arcticum lappa L.) roots by liquid chromatography tandem mass spectrometry, R. Jaiswal and N. Kuhnert, Food and Function manuscript accepted.read online
-
Scope and limitations of principal component analysis of high resolution LC-MS data: The analysis of the chlorogenic acid fraction in green coffee beans as a case study, N. Kuhnert, R. Jaiswal, PO. Eruvichera, M. El-Abassy, B. von der Kammer and A. Materny, Anal. Manuscript accepted. read online
-
Synthesis of Tri-substituted Biaryl Based Trianglimines: Formation of C3-symmetrical and Non-symmetrical Regioisomers, H. F. Nour, M. F. Matei, B. S. Bassil, U. Kortz and N. Kuhnert. Manuscript accepted 2010.read online
-
Hill Coefficient of dietary polyphenolic enzyme inhibitors: Can beneficial health effects of dietary polyphenols be explained by allosteric enzyme denaturing? N. Kuhnert, F. Dairpoosh, R. Jaiswal, M. Matei, S. Deshpande, A. Golon, H. Nour, H. Karakoese and N. Hourani, Journal of Chemical Biology, 2010 (Submitted). read online
-
An investigation of the Hydroxycinnamate Profile of Six Galium Plants from the Rubiaceae Family. R. Jaiswal, S. Deshpande and N. Kuhnert. Phytochemical Analysis, 2010, (Submitted).
-
How to Identify and Discriminate Between the Methyl Quinates of Chlorogenic Acids by Liquid Chromatography/Tandem Mass Spectrometry. R. Jaiswail and N. Kuhnert. Journal of Mass Spectrometry, 2010, (Submitted). read online
-
Profiling the Hydroxycinnamates of 12 Plants from Asteraceae Family by High–Performance Liquid Chromatography/Tandem Mass Spectrometry. R. Jaiswal, J. Kiprotick and N. Kuhnert. Phytochemistry, 2010, (Submitted).
-
Identification and Characterization of Two New Classes of Chlorogenic Acids in Arnica (Arnica montana L.) Flowers by High–Performance Liquid Chromatograpghy. R. Jaiswal and N. Kuhnert. Journal of Agricultural and Food Chemistry, 2010, (Submitted).
-
“Identification and characterization of five new classes of chlorogenic acids containing aliphatic side chains in Burdock (Arcticum lappa L.) roots by liquid chromatography tandem mass spectrometry” R. Jaiswal and N. Kuhnert, Food and Function 2011, 2, 63-71.
-
“Scope and limitations of principal component analysis of high resolution LC-MS data: The analysis of the chlorogenic acid fraction in green coffee beans as a case study” N. Kuhnert, R. Jaiswal, PO. Eruvichera, M. El-Abassy, B. von der Kammer and A. Materny, Anal. Meth. 2011, 3, 144-155.
-
“Synthesis of substituted biaryl based trianglimine and trianglamine mecrocycles: Formation of C3symmetrical versus non-symmetrical macrocycles” H. Nour, M. Matei, B. Bassil, U. Kortz, N. Kuhnert, Org. Biomol. Chem. 2011, 9, 3258-3271.
-
“Characterisation of chlorogenic acids in Galium species” R. Jaiswal, N. Kuhnert, Phytochem. Anal.manuscript accepted.
-
“Identification of all Regioisomers of Methylquinates by LC-tandem mass spectrometry” R. Jaiswal, N.Kuhnert, J. Mass Spectrom. 2011, 46, 269-281.
-
“Schwarzer Tee –Der Meister der molekularen Vielfalt”,N. Kuhnert, Laboratory and More, 2011, 124-128.
-
“Hill coefficients of dietary Polyphenols – Are dietary polyphenols nothing else but allosteric enzyme denaturing agents?”, N. Kuhnert, F. Dairpoosh, H. Nour, N. Hourani, R. Jaiswal, M. Matei, S. Deshpande, A. Golon, H. Karaköse, J. Chem. Biol. 2011, 21, 755-763.
-
“Profiling the Hydroxycinnamates of 12 Plants from Asteraceae Family by High-Performance Liquid Chromatography/Tandem Mass Spectrometry”, R. Jaiswal, J. Kiprotich, N. Kuhnert, Phytochem.2011, 72, 781-790.
-
“Profiling the chlorogenic acids in Arnica Montana flowers: Identification of five new classes of aliphatic chlorogenic acids” R. Jaiswal, N. Kuhnert, J. Agr. Food Chem. 2011, 59, 4033-4039.
-
“Inhibition of DNA Methyltranferase 3a by black tea and coffee phenolics – a potential mechanism for the brain performance enhancing effects of dietary phenols” A. Tulysheva, R. Jaiswal, N. Kuhnert, A. Jeltsch, BMC Biochemistry, 2011, DOI: 10.1186/1471-2091-12-16.
-
“Characterisation of dehydration products of chlorogenic acids in roasted coffee” M. F. Matei, R. Jaiswal, F. Ullrich and N. Kuhnert, J. Mass Spectrom. 2011, manuscript accepted.
-
“Assessing the thearubigins from black tea by high resolution MS methods” N. Kuhnert, Deutsche Lebensmittel Rundschau 2011, 107, 388-391.
-
“Was steckt unter dem Hügel?”N. Kuhnert, Nachr. Chemie und Technik 2011, 866-871.
-
„Profiling and quantification of the chlorogenic acids in Stevia Rebaudiana”, H. Karaköse, R. Jaiswal, N.Kuhnert, J. Agr. Food Chem. 2011, 59, 10143-10150.
-
“An analytical method for the discrimination of caffeoyl shikimates and caffeoyl quinides in heat processed food”, N. Kuhnert, M. F. Matei,F. Ullrich, N. Kuhnert, J. Mass Spectrom.2011, 46, 933-942.
-
“Regioisomere Chlorogensäurederivate- Identifizierung durch Tandem Massenspektrometrie”, N.Kuhnert, Deutsche Lebensmittelrundschau 2011, 107, 521-524.
-
“Synthesis of novel enantiomerically pure tetra-carbohydrazide cyclophane macrocycles“ H. F. Nour, N. Hourani, Org. Biomol. Chem. 2012, 10, 4381-4389.
-
“First diastereoselective synthesis of caffeoyl and feruloyl muco-methylquinates” R. Jaiswal, M. H. Dickman,N. Kuhnert, Org. Biomol. Chem. 2012, 10, 5266-5277.
-
“Chemistry of pyrazolinones and their applications” WH. Wafaa, H. G. El-Gohary, N. Kuhnert, Current Org. Chem. 2012, 3, 373-399.
-
“Development of a novel direct infusion atmospheric pressure chemical ionization mass spectrometry method for the analysis of heavy hydrocarbons in light shredder waste” N. Hourani, N. Kuhnert, Anal. Meth.2012, 3, 730-735.
-
“Monitoring stepwise proteolytic degradation of peptides using supramolecular domino tandem assays and mass spectrometry for trypsin and leucine aminopeptidase” G. Ghale, N. Kuhnert, W. M. Nau, Nat. Prod. Commun. 2012, 7, 343-348.
-
“Black tea – the ultimate master of molecular diversity” Lab and More Int. Ed. Engl. 2012, 465-467.
-
“Unravelling the chemical composition of caramel”, A. Golon, N. Kuhnert, J. Agr. Food Chem. 2012, 60, 3266-3274.
-
“Analyse von Karamel durch Domino Tandem Massenspektrometrie” A. Golon, N. Kuhnert, Deutsch.Lebensmittelrundschau 2012, 108, 148-151.
-
“Identification and characterization of proanthocyanidines of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS” R. Jaiswal, L. Jayasinghe, N. Kuhnert, J. Mass Spectrom. 2012, 47, 502-515.
-
“MALDI-TOF mass spectrometry: avoidance of artifacts and analysis of caffeine precipitated S II thearubigin fraction from 15 commercial black teas” J. W. Drynan, M. N. Clifford, J. Obuchowicz, N. Kuhnert, J. Agr. Food Chem. 2012, 60, 4514-4525.
-
“Probing the dynamic reversibility of trianglimine formation using real time mass ESI time of flight mass spectrometry” H. F. Nour, A. Lopez-Periago, N. Kuhnert, Rap. Commun. Mass Spectrom. 2012, 26, 1070-1080.
-
“Raman spectroscopic characterization of different regioisomers of mono acyl and di acyl chlorogenic acids” P. Eravuchira, R. M. El-Abassy, S. Deshpande, N. Kuhnert, A. Materny, Vib. Spectr. 2012, 61, 10-16.
-
“Polyphenole in Lebensmitteln, Arzneidrogen und industrieller Anwendung”ChemiuZ, 2012, manuscript accepted.
-
“Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies” R. Jaiswal, M. F. Matei, A. Golon, M. Witt, N. Kuhnert, Food Func. 2012,3, 976-984.
-
“Synthesis of enantiomerically pure tetra carbohydrazide cyclophane macrocycles” H. F. Nour, N. Hourani,N. Kuhnert, Org. Biomol. Chem. 2012, 10, 4381-4389.
-
“First diastereoselective synthesis of methyl caffeoyl and feruloyl muco quinates” R. Jaiswal, M. H. Dickman, N. Kuhnert, Org. Biomol. Chem. 2012, 10, 5266-5277.
-
“Investigating the chemical changes of chlorogenic acids during coffee brewing: Conjugate addition of water to the olefinic moiety of chlorogenic acids and their quinides” M. F. Matei, R. Jaiswal, N. Kuhnert, J. Agr. Food Chem. 2012, 60, 12105-12115.
-
“Lessons from the analysis of complex dietary mixtures by mass spectrometry – understanding the chemistry of black tea thearubigins, coffee melanoidines and caramel”, N. Kuhnert, F. Dairpoosh, A. Golon, G. Yassin and R. Jaiswal, Food and Function, 2013,4, 1130-1147.
-
“In Garden, Industry Medicine and Food: Polyphenols versatile Plant Ingredients”, N. Kuhnert, Chemie in Uns. Zeit, 2013, 47, 80-91.
-
“Characterisation of caramel – type thermal decomposition products of monosaccharides including glucose, mannose, galactose, arabinose and ribose using advanced mass spectrometrical methods” A. Golon and Kuhnert, Food Function, Maillard centenary edition, 2013, 4, 1040-1050.
-
“Analysis of chlorogenic acid lactones and caffeoyl shikimic acids in roasted coffee”, Eng. Food Sci. 2013, 17, 568-576.
-
“Chemistry inside molecular containers in the gas phase” C. Lee, E. Kalenius, A. I. Lazar, I. Assaf, N. Kuhnert, C. H. Grün, J. Jänis, O. A. Sherman, W. N. Nau, Nature Chemistry, 2013, 4, 376-382.
-
“One size does not fit all – bacterial cell death by antibiotics cannot be explained by the action of reactive oxygen species”, N. Kuhnert, Chem. Int. Ed. Engl. 2013, 52, 10946-10948.
-
“Identification of Phenolic Compounds in Plum Fruits (Prunus salicina L. and Prunus domestica L.) by High-Performance Liquid Chromatography/Tandem Mass Spectrometry and Characterization of Varieties by Quantitative Phenolic Fingerprints” R. Jaiswal, H. Karaköse, S. Ruehmann, D. Treutter and N. Kuhnert, Agr. Food Chem. 2013, 61, 1220-1231.
-
“Synthesis of novel chiral bis-N-substituted-hydrazinecarboxamide receptors and probing their solution-phase recognition to chiral carboxylic guests by ESI-TOF/MS and tandem ESI-MS” H. Nour, A. Golon, T. Islam, M. Fernandez-Lahore, N. Kuhnert, Tetrahedron Lett. 2013, 54, 4139-4142.
- “Identification and Characterization of the Phenolic Glycosides of Lagenaria siceraria Stand. (Bottle Gourd) Fruit by Liquid Chromatography-Tandem Mass Spectrometry” R. Jaiswal, N. Kuhnert, Food Chem. 2014, 62, 1261-1271.
- “Biologische Reststoffbehandlung bei der Autoverwertung” D. Lompe, C. Schubert, J. Warrelmann, A. Groddek, Hewitter, A. Golon and N. Kuhnert, Müll und Abfall, 2014, 14, 259-268.
- “Identification, Characterization, Isolation and Activity Against Escherichia coli of Quince (Cydonia oblonga) Fruit Polyphenols” M. G. Elsadig Karar, D. Pletzer, R. Jaiswal, H. Weingart, N. Kuhnert, Food Res. Int. 2014, 65, 121-129.
- “Which spectroscopic technique allows the best differentiation of coffee varieties: comparing principal component analysis using data derived from CD-, NMR- and IR-spectroscopies and LC-MS in the analysis of the chlorogenic acid fraction in green coffee beans”, S. Deshpande, R. M. El-Abassy, R. Jaiswal, P. Eravuchira, B. von der Kammer, A. Materny and N. Kuhnert, Analytical Methods 2014, 6, 3268-3276.
- “Understanding the dark side of food: analysis of processed food by modern mass spectrometry” N. Kuhnert, New Food 2014, 17, 58-64.
- “ Identification of novel cocoa flavonoids from raw fermented cocoa beans by HPLC-MS “, M. A. Patras, B. Milev, G. Vrancken, Food Res. Int. 2014, 63, 353-359.
- “Fourier transform ion cyclotrone resonance mass spectrometrical analysis of raw fermented cocoa beans from Cameroon and Ivory Coast origin” B. Milev, M. A. Patras, G. Vrancken, N. Kuhnert, Food Res. Int. 2014, 64, 958-962.
- “An Investigation of the complexity of Maillard reaction product profiles from the thermal reaction of amino acids with sucrose using high resolution mass spectrometry”, A. Golon, I. Vockenroth, C. Kropf, N. Kuhnert, Food, 2014, 3, 461-475.
- “Identification of trimeric and tertameric flavan-3-ol derivatives in the SII black tea thearubigin fraction of black tea using ESI-Tandem and MALDI-TOF Mass Spectrometry”, G. H. Yassin, J. Koek, N. Kuhnert, Food Res. Int. 2014, 63, 317-327.
- “Hierarchical key for the identification of all ten regio- and stereoisomers of monocaffeoyl glucose by LC-tandem mass spectrometry”, R. Jaiswal, M. A. Patras, M. Matei, V. Glembockyte, N. Kuhnert, Food Chem. 2014, 62, 9252-9265.
- “Investigation of isomeric flavanol structures in black tea thearubigins using UHPLC coupled to ion mobility mass spectrometry” G. H. Yassin, J. Koek, N. Kuhnert, Mass Spectrom. 2014, manuscript in press.
- “LC-MSn identification and characterization of the phenolic compounds from the fruits of Flacourtia indica (Burm. F.) Merr. and Flacourtia inermis Roxb” A. G. A. W. Alakolanga, A. M. D. A. Siriwardane, N. S. Kumar, L. Jayasinghe, R. Jaiswal and N. Kuhnert, Food Res. Int. 2014, 62, 388-396.
- “Does roasted coffee contain chlorogenic acid lactones and/or caffeoyl shikimates?”, R. Jaiswal, M. F. Matei and N. Kuhnert, Food Res. Int. 2014, 61, 214-227.
- “Investigation of acyl migration in mono- and dicaffeoylquinic acids under aqueous basic, acidic and dry roasting conditions” Chem. 2014, 62, 9160-9170.
- “Identification of Novel Homologous Series of Polyhydroxylated Theasinensins and Theanaphthoquinones in the SII Fraction of Black Tea Thearubigins using ESI /HPLC Tandem Mass Spectrometry” G. H. Yassin, J. Koek, S. Jayaraman and N. Kuhnert, Food Chem. 2014, 62, 9848-9859.
- “Identification and characterization of phenolics of Ilex Glabra (Aquifoliaceae) leaves by liquid chromatography mass spectrometry” R. Jaiswal, A. E. Halabi, M. G. E. Karar and N. Kuhnert, 2014, 106, 141-155.
- “Identification and characterization of phenolics from Ixora coccinea (Rubiaceae) by liquid chromatography multi stage mass spectrometry” R. Jaiswal, M. G. Elsadig Karar, G. H. Abdel, and N. Kuhnert, 2014, 25, 567-576.
- “Investigation of isomeric flavanol structures in black tea thearubigins using ultraperformance liquid chromatography coupled to hybrid quadrupole ion mobility time of flight mass spectrometry” G. H. Yassin, C. Grün, J. Koek and N. Kuhnert, Mass Spectrom. 2014, 49, 1086-1095.
- “Identification, characterization and antimicrobial activity of quince (Cydonia oblongata) fruit polyphenols” M. G. E. Karar, R. Jaiswal and N. Kuhnert, Food Res. Int. 2014, 65, 121-129.
- “Identification and Characterization of Trimeric Proanthocyanidins of Two Members of the Rhododendron Genus (Ericaceae) by Liquid Chromatography Multi‐Stage Mass Spectrometry” R. Jaiswal, M. G. E. Karar and N. Kuhnert. Encyclopedia of Analytical Chemistry. Marietta: Wiley Online Library, 2014.
- “Studies in Natural Products Chemistry” N. Kuhnert, I. Hakeem Said and R. Jaiswal, ed. R. Atta ur, Elsevier, 2014, vol. 42, pp. 305-339.
- “Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera Henry L. (Caprifoliaceae) leaves by LC-MSn”. R. Jaiswal, H. Müller, A. Müller, M. G. E. Karar and N. Kuhnert, Phytochem.2014, 108, 252-263.
-
Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn, M. Clifford, S. Knight and N. Kuhnert, Agr. Food Chem.2005, 53, 3821-3832. read online
-
Characterization by LC–MSn of four new classes of chlorogenic acid methyl ethers in green coffee beans: dimethoxycinnamoylquinic acids, diferuloylquinic acids, caffeoyl-dimethoxycinnamoylquinic acids, and feruloyl-dimethoxycinnamoylquinic acids“ M. Clifford, S. Knight and N. Kuhnert, Agr. Food Chem. 2005,53, 3461-3471.
-
The synthesis and conformational properties of oxygenated trianglimine macrocycles, N. Kuhnert, A. Lopez-Periago and G. M. Rossignolo, Biomol. Chem. 2005, 3, 524-537. read online
-
The synthesis of static and dynamic combinatorial libraries using deep cavity tetra-formyl calix[4]arenes, N. Kuhnert and A. Le-Gresley, Tetrahedron Lett.2005,46, 2059-2062.
-
Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: implications for its chemopreventive activity, V. Yoxall, P. Kentish, N. Coldham, N. Kuhnert, M. J Sauer1 and C. Ioannides, J. Cancer. 2005, 1354-1368.read online
-
On the activation of valerolactam using triflic anhydride, N. Kuhnert, I. Clemens, R. Walsh, Biomol. Chem.2005, 3, 1694 – 1701. read online
-
Varying the size of the trianglimine cavity-Macrocycles for applications in nanomachines, N. Kuhnert, C. Patel, N. Burzlaff and A. Lopez-Periago, Biomol. Chem.2005, 3, 1911-1921.
-
Synthesis and capsule formation of tetra-acrylamido calix[4]arenes, N. Kuhnert and A. Le-Gresley, Biomol. Chem. 2005, 3, 2175-2182. read online
-
Synthesis of chiral non-racemic polyimine macrocycles from cyclocondensation reactions of biaryl and terphenyl based dicarboxaldehydes and 1R, 2R diaminocyclohexane, N. Kuhnert, C. Patel and F. Jami.,Tetrahedron Lett.2005, 46, 7575-7579. read online
-
Total synthesis of chrysophanol and sennoside C aglycon, N. Kuhnert and H.Y. Molod, Tetrahedron Lett.2005, 46, 7571-7573. read online
-
Characterisation by LC-MSn of four new classes of chlorogenic acids in green coffee beans: dimethoxycinnamoylquinic acids, diferuloylquinic acids, caffeoyl-dimethoxycinnamoylquinic acids and feruloyl-dimethoxycinnamoylquinic acids, M. N. Clifford, S. Knight, B. Surucu, N. Kuhnert, Agr. Food. Chem. 2006, 54, 1957-1969. read online
-
Synthesis of diastereomeric trianglamine cyclodextrin [2] catenanes, N. Kuhnert and B. Tang, Tetrahedron Lett.2006, 47, 2985-2988. read online
-
Synthesis of enantiomerically pure functionalized trianglamine macrocycles by N-acylation and N-alkylation reactions, N. Kuhnert, D. Goebel, C. Thiele, B. Tang, Tetrahedron Lett.2006, 47, 6915-6918. read online
-
Characterisation by LC-MSn of four new classes of p-coumaric acid containing diacyl chlorogenic acids in green coffee beans”, M. N. Clifford, S. Marks, S. Knight, N. Kuhnert, Agr. Food Chem. 2006, 54, 4095-4101. read online
-
The chlorogenic acids of Hemerocallis, M. N. Clifford, W. Wu and N. Kuhnert, Food Chem. 2006, 95, 574-578. read online
-
Profiling the chlorogenic acids of aster by HPLC-MSn, M. N. Clifford, Z. Wang, N. Kuhnert, Anal. 2006, 17, 384-393. read online
-
Profiling the chlorogenic acids and other caffeic acid derivatives of herbal chrysanthemum by LC-MSn, M. N. Clifford,W. Wu, J. Kirkpatrick, N. Kuhnert, Agr. Food Chem. 2007, 55, 929-936. read online
-
Profiling and characterization of galloyl quinic acids by LC-MSn of green tea, tara tannin and tannic acid, M.N. Clifford, S. Stoupi, N. Kuhnert, Agr. Food. Chem.2007, 55, 2797-2807. read online
-
A structural model for the ion suppression effects of carboxylic acids in negative ESI ion mode, M. N. Clifford, L. Pouquet, V. Lopez, G. Williamson , N. Kuhnert, Commun. Mass Spectr. 2007, 21, 2014-2018. read online
-
The application of quasi-enantiomeric trianglamine macrocycles as chiral probes for anion recognition in ion trap ESI mass spectrometry, N. Kuhnert, D. Marsh, D. C. Nicolau, Tetrahedron Asymm. 2007, 18, 1648-1654. read online
-
The synthesis, self-association and chiroselectivity of isotopically labeled trianglamine macrocycles in the ion trap mass spectrometer, N. Kuhnert, A. Le-Gresley, D. C. Nicolau, Labelled Comp. Radiopharm.2007, 50, 1215-1223. read online
-
The metabolism of 2-amino.3.methylimidazol [4,5] quinoline by precision-cut rat liver slices, N. Arya, N. Kuhnert, C. Ioannides, J. Kirkpatrick, D. Stevenson, Toxicology2007, 240, 185.
-
The absolute bioavailability and dose dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat, N. Hanlon, N. Coldham, A. Gielbert, M. Sauer, N. Kuhnert, C. Ioannides, British J. Nutrition2008, 99, 559-564. read online
-
Synthesis of upper rim calix[4]arene carcerands, N. Kuhnert, A. Le-Gresley, Tetrahedron Lett. 2008, 49, 1274-1276. read online
-
The LC-MSn analysis of cis isomers of chlorogenic acids, N. Kuhnert, M. N. Clifford, Food Chem. 2008, 106, 379-385. read online
-
Insecticidal activities of Zingiber officinalis and cynbopogon nardus, Z. Abo Elnaga, N. Kuhnert, M. Abdel-Mogib, Pharmacetical Biol. 2009, 47, 27.
-
Stereo- and Enantioselective Reactions of Organosulfur Compounds Mediated by Transition Metal Complexes, W. A. Schenk, J. Bezler, N. Burzlaff, E. Dombrowski, J. Frisch, N. Kuhnert, I. Reuther, Phosphor Sulfur Silicon Rel. Elem.1994, 95, 367. read it
-
Stereochemically Controlled Synthesis of Substituted 1,2 Oxathianes, J. Eames, N. Kuhnert, R. V. H. Jones and S: Warren, Tetrahedron Lett. 1998, 39, 1251-1254. read it
-
Scope and Limitation of [1,4]-S-Benzyl Participation and Debenzylation in the Stereochemically Controlled Synthesis of Substituted Thiolanes, J. Eames, N. Kuhnert, R. V. H. Jones and S, Warren, Tetrahedron Lett.1998,39, 1247-1250. read it
-
Bis-Trifluoromethansulfonimide in the Catalytic Conjugate Allylation of alpha,beta-Unsaturated Carbonyl Compounds, N. Kuhnert, J. Peverley and J. Robertson, Tetrahedron Lett. 1998, 39, 3215-3216. read it
-
Kinetic Versus Thermodynamic Control in the Stereospecific Synthesis of Tetrahydro-Pyrans and Furans: Exo Versus Endo Transition States and [1,2] Versus [1,4] Phenylsulfanyl Participation, J. Eames, N. Kuhnert and S. Warren, Synlett 1999,8, 1211-1214.
-
[1,2]- and [1,4] Phenylsulfanyl Migrations as Alternative Cascade Sequences for the Stereocontrolled Synthesis of Tetrahydrofurans, J. Eames, N. Kuhnert and S. Warren, Synlett 1999, 8, 1215-1218.
-
Acetylsalicylsäure feiert Ihren 100. Geburtstag, Chemie in unserer Zeit 1999,33, 213-220.
-
Aspirin, das erfolgreichste Arzneimittel des letzten Jahrtausends, Pharmazie in unserer Zeit 2000, 14, 1-8.
-
Halfsandwich Ruthenium Complexes of Sulfinic Esters, N. Kuhnert and W. A. Schenk, Naturforsch B 2000, 55, 527 – 535. read online
-
1282 Chemische Bachelor und Master-Studiengänge in Grossbritannien, N. Kuhnert, Nachrichten aus Chem. und Technik 2000, 11, 1352-1356. read online
-
Synthesis of 14-C-Labelled Sulforaphane, N. Kuhnert, G. Williamson and B. Holst, Labelled Comp. Radiopharm.2001, 44, 347-355. read online
-
Synthesis of 3-Chloro-3-Formylpyrrole Derivatives, J. Robertson, N. Kuhnert and Y. Zhao, Heterocycles2000, 53, 2415-2420.
-
Scope and Limitation of the [1,2]-Alkylsulfanyl (SMe, SEt and SCH2Ph) and Sulfanyl (SH) Migration in the Stereospecific Synthesis of Substituted Tetrahydrofurans, J. Eames, N. Kuhnert and S. Warren, Chem. Soc. Perkin Trans. 12001, 138-143. read online
-
Highly Diastereoselective Synthesis of Homochiral 1,3-Oxazolidines under Thermodynamic Control Using Focused Microwave Irradiation under Solvent Free Conditions, N. Kuhnert and T. N. Danks, Green Chem.2001, 3, 68-70. read online
-
Unkonventionell Techniken in der Organischen Synthese, N. Kuhnert, Chemie und Technik 2001(Trendberichte), 306-310. 17.
-
Microwave Accelerated Synthesis of Cyclopentadienyl Bis-Phosphine Ruthenium Thiolato Complexes Using Focused Microwave Irradiation, N. Kuhnert and T. N. Danks, Chem. Res. (S) 2002, 66-68. read online
-
Scope and Limitation of [1,4]-S-Benzyl Participation and Debenzylation in the Stereochemically Controlled Synthesis of Substituted Thiolanes, J. Eames, N. Kuhnert and S. Warren, Chem. Soc. Perkin Trans. 1,2001, 1504-1511. read online
-
Boron-Trifluoride Mediated Synthesis of 2-Deoxy-anthocyanidines including a Total Synthesis of Tricetanidin from Black Tea (Camilla Sinensis), N. Kuhnert, M. Clifford and A.-G. Radenac, Tetrahedron Lett.2001, 42, 9261-9264.
-
Scope and Limitation of the Heck Reaction of Upper Rim Substituted Tetraiodo Calix[4]-arenes, N. Kuhnert and A. Le-Gresley, Chem. Soc. Perkin Trans. 12001, 3393- 3398. read online
-
Synthesis, Reactivity, Structure and Dynamic Behaviour of Ruthenium Sulfine Complexes, N. Kuhnert, N. Burzlaff, E. Dombrowski uns W. A. Schenk, Naturforsch B2002, 57, 259-274.
-
Synthesis of Enantiomerically Pure 42 and 30 Membered Ring Trianglimine and Tranglamine Macrocycles, N. Kuhnert, C. Straßnig and A. M. Lopez-Periago, Tetrahedron Asymm.2002, 13, 123-128.
-
Synthesis of Chiral Non-Racemic Macrocycles of the Trianglimine and Trianglamine Type, N. Kuhnert und A. Lopez-Periago, Tetrahedron Lett.2002, 43, 3329-3332. read online
-
Is There a Special Non-Thermal Microwave Effect, N. Kuhnert, Chem. Int. Ed. Engl. 2002, 41, 1863-1866.
-
Gibt es einen nicht-thermischen Mikrowelleneffekt?, N. Kuhnert, Chem.2002, 114, 1943-1946.read online
-
Microwave Accelerated Synthesis of Cyclopentadienyl Bis-Phosphine Ruthenium Thiolato Complexes Using Focused Microwave Irradiation, N. Kuhnert and T. N. Danks, Met. Rev. 2002, 46, 140 (abstract).
-
Stereochemically Controlled Synthesis of Substituted 1,2 Oxathianes, J. Eames, N. Kuhnert and S. Warren, Chem. Soc. Perkin Trans. 12002, 2282-2287. read online
-
Funktionelle Lebensmittel-Eine Kritische Betrachtung, N. Kuhnert, Chemie und Technik 2002, 50, 142-147. read online
-
On the Steric Acceleration of Ene Reactions, N. Chooney, N. Kuhnert, P. G. Sammes, G. Smith, R. W. Ward, Chem. Soc. Perkin Trans. 12002, 1999-2006. read online
-
Hierarchical Scheme for the LC–MSn identification of chlorogenic acids, M. N. Clifford, K. L. Johnston, S. Knight and N. Kuhnert, Agr. Food. Chem.2003, 51, 2900-2911. read online
-
On the Scope and Limitations of the [3+3] Cyclocondensation Reaction of of Aromatic Dicarbonyl Compounds and 1R, 2R-cyclohexanediamine, N. Kuhnert, A. Lopez-Periago and G. M. Rossignolo, Biomol. Chem.2003, 1, 1157 – 1170. read online
-
On the Synthesis of Upper Rim Tetraacrylamidocalix[4]arenes and their Dimerisation to Form Molecular Capsules Using Eight Hydrogen Bonds, N. Kuhnert and A. Le-Gresley, Commun.2003, 2426-2427.read online
-
Detection and Imaging of Vibrationally Labelled Biologically Active Compounds in Living Cells Using Raman Microscopy, N. Kuhnert, A. Thumser, Labl. Comp. Radiopharm.2004, 47, 493-500.
-
The synthesis of 1,1’,2,2’,3,3’,4,4,’-Octadetero-Sulforaphane, N. Kuhnert and Y. Lu, Labl. Comp. Radiopharm. 2004, 47, 501-507.
-
A powerful aqueous solvent effect in an intramolecular Diels-Alder cyclisation, N. Kuhnert, P.G. Sammes, G. Smith and R. W. Ward, Chem. Res.2004, 608-610.
- Postdoc
- PhD Student
- Research Assistant
- Technician
- PhD Chemistry Student

Phytoextracts Characterization and Sustainable Formulation of Aerosol-based Disinfectants from Food Production Waste
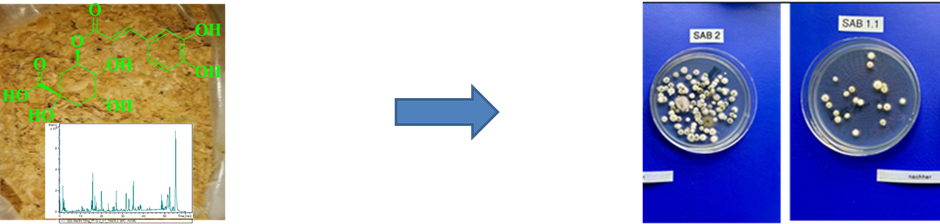
Most food waste is discarded at various stages in the food production chain, underscoring the need for sustainable utilization to mitigate environmental hazards. Bioactive phytoextracts derived from these wastes offer alternatives to hazardous synthetic chemicals. My research involves extraction, isolation, and purification of bioactive compounds from food byproducts and testing their antimicrobial efficacy against Listeria, Vibrio, and formulations of aerosol-based disinfectants against surfaces and airborne microbes. Comprehensive chemical characterization methods like LC-QTOF-MS, 1H-NMR, and FTIR will be employed to unravel the complex bioactive compounds in the phytoextracts. This initiative lays the groundwork for further exploitation of waste for green chemical production in the food, pharmaceutical, and hygiene industries, marking a new paradigm in these fields[1–3].
[1] I. H. Said and N. Kuhnert, Plant Phenolics as an Alternative Source of Antimicrobial Compounds, 4th ed. Herbal Medicine: Back to the Future, 2021.
[2] S. Mahfooz and M. Itrat, “Effect of Plant-Products Fumigation on Air-borne Microbes Effect of Plant-Products Fumigation on Air-Borne Microbes,” no. November, 2021, doi: 10.9734/jpri/2021/v33i49A33312.
[3] T. Sandle, “Disinfection and Biodides,” in Disinfection and Decontamination: A Practical Handbook, 2018, p. 7.
-
Technician
I’m one of the Technicians in the lab.
I started my apprenticeship in 2008 at the Alfred-Wegener Institute after finishing in 2011 I started in the Biogeochemistry Group.
Since 2023 I have been part of the Kuhnert Group at the Constructor University. In the workgroup, I’m responsible for the instruments' maintenance and technical support. I’m also involved in teaching undergraduate lab courses, namely “Advanced Integrated Organic and Analytical Chemistry Lab” [Fall Semester for 2nd Year B.Sc. students]
When I’m not on Campus I spend most of my time outside in the nature in our garden in Bremen or spending time with my horse.
- PhD Student
- Dr. Robert Lucas [United Kingdom]
- Dr. Adam Le Gresley [United Kingdom]
- Dr. Ana Lopez-Periago [Spain]
- Dr. Giulia Rossignolo [Italy]
- Dr. Hoshiar Molod [Iraq]
- Dr. Rodney Walsh [United Kingdom]
- Dr. Fatemeh Jami [Iran]
- Dr. Warren Drynan [Zimbabwe]
- Dr. Rakesh Jaiswal [India]
- Dr. Birgul Surucu [Turkey]
- Dr. Farnoosh Dairpoosh [Iran]
- Dr. Nadim Hourani [Lebanon]
- Dr. Hany Nour [Egypt]
- Dr. Agnieszka Golon [Poland]
- Dr. Hande Karaköse [Turkey]
- Dr. Sagar Anil Deshpande [India]
- Dr. Mohamed Elsadig [Sudan]
- Dr. Michael Sanguinetti [USA] (joint with Prof. Dr. Laurenz Thomsen)
- Dr. Marius-Febi Matei [Romania]
- Dr. Abhinandan Shrestha [Nepal]
- Dr. Rohan Shah [India]
- Dr. Inamullah Hakeem Said [Afghanistan]
- Dr. Maria Alexandra Patras [Romania]
- Dr. Diana Sirbu [Moldova]
- Dr. Seung-Hun Lee [South Korea]
- Dr. Roberto Megias [Spain]
- Dr. Maria Bikaki [Greece]
- Dr. Pawel Andruszkiewicz [Poland]
- Dr. Anastasiia Shevchuk [Ukraine]
- Dr. Sabur Olarotimi Badmos [Nigeria]
- Dr. Tahira Mussarat [Pakistan]
- Dr. Fariba Sabzi [Iran]
- Dr. Salma Elshami [Egypt]
- Dr. Achala Alakolanga [Sri Lanka]
- Dr. James Ziemah [Ghana]
Guest Scientists
- Prof. Dr. Edwin Ntakadenzi Madala [South Africa]
- Prof. Dr. Adeola Sonibare [Nigeria]
- Prof. Dr. Lalith Jayasinghe [Sri Lanka]
- Dr. Hiba Ali [Sudan]
- Dr. Zenab [Egypt]
Former Master Students
- Audrey Bergounhou [France]
- Nickolet Ncube [Zimbabwe]
- Joanna Dabrowska [Poland]
- Lucas Lansing [Germany]
- Borislav Milev [Bulgaria]
- Kianoosh Dairpoosh [Iran]
- Awa Rita Diallo [Ivory Coast]
Former Bachelor Students
- Victoria von Glasenapp [Germany]
- Megi Mustafai [Albania]
- Naika Thielen [Germany]
- Rachelle Smith [USA]
- Gediminas Mikutis [Lithuania]
- Viktorija Glembockytė [Lithuania]
- Tina Sovdat [Slovenia]
- Prabal Subedi [Nepal]
- Joseph Kiprotich [Kenya]
- Sanan Eminov [Turkey]
- Fransesco Vivian [Italy]
- Marcus Papmeyer [Germany]
- Warren Andrew [Sri Lanka]
- Selin Gencer [Turkey]
- Irem Altun [Turkey]
- Nicoleta Copaci [Moldova]
- Yeweynwuha Gellaw Zemedie [Ethiopia]
- Nicolescu Vlad-Alexandru [Romania]
- Lingyi Jiao [China]
- Christina Heidorn [Germany]
- Maotian Fu [China]
- Johnathan Douglas Truex [USA]
- Mihella Berhanu Retta [Ethiopia]
- Sara Haka [Albania]
- Dimitar Dimitrov Petrov [Bulgaria]
- Nikesh Chandra Bhardwaj [Germany]
- Tsion Berhane Woldermariam [Ethiopia]
- Argi Pesha [Albania]
- Paula Cotrell [UK]
- Nikolas Ohl [Germany]
- Maryam Mahjoub [Iran]
- Mobinasadat Sharifi [Egypt]
- Sanjeeb Panthi [Nepal]
- Maria Santos [Guatemala]
- Sang Jin [South Korea]
- Mikhael Astorga [Phillipines]
- Munktuul Enkhbat [Mongolia]
- Megan Gurra [Albania]
- Jera Rexhepi [Albania]
- Elena Vide [Albania]
- Yordanos Abeje [Ethiopia]
- George Jabishvili [Georgia]
- Zhibek Konsbayeva [Kazachstan]
- Martinaiden Demaij [Albania]
- Burat Bürgec [Turkey]
- Hiba Mastas [Marocco]
- Yahann Amissah [Ghana]
- Nayana Upadyya [Nepal]
- Hillary Baddoo [Ghana]
- Juan Oviedo [Ecuador]




Currently there are no vacancies for PhD positions in my group.

aq




